Sulfate Attack – What Why Avoid
Doing construction and avoiding sulfate attack in an area having sulfate in the ground is a real challenge. The sulfate resistance of a structure can be achieved by different means as discussed in this article.
Knowing the factors to be considered during the design and the construction is very important for engineers to make a durable and safe structure.
Let’s discuss little things but important to have knowledge…
What are the sources of sulfate?
Sulfate can exist in the soil and groundwater. It could exist due to the following reasons.
- Naturally
- Wast from chemical and mining industry
- Industrial effluents
- Seawater
Having sulfate in the seawater is quite common and other forms of sources are not observed always.
How to identify Sulfate?
Sulfate dilutes in the water even if it contains in the ground when the water table is in contact with such soils.
It the common practice to carrying out soil investigation before the structural designs to obtain the soil parameters and to obtain the type of foundation with geotechnical design data.
However, as observed, sometimes, engineers do not tend to test the groundwater to make sure there is no harmful material affecting the durability of concrete though it is a very important test to be done.
The types of tests that need to be done in relation to the water are discussed in the article Factors Affecting Concrete Mix Design.
What is a Sulfate Attack?
Sulfate attack is the reaction of the sulfate ions with the concrete and producing new formation within the concrete.
As a result, the combination of chemical compositions is created and they tend to increase the internal volume of the concrete leading to internal stresses.
Such expansions cause cracking, loss of strength of concrete, loss of elastic properties, etc.
Further, sulfate attack can be identified as another mode of deterioration of concrete. The damage level depends on the content of sulfate that the structure is exposed to.
Mainly, three-stage of sulfate attacks are identified.
- Ettringite Formation
- Gypsum Formation
- Reaction of Magnesium Ions
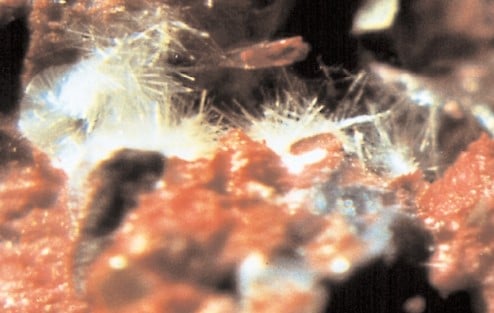
Ettringite Formation
It quite a popular term that is the formation of delayed ettringite in the concrete due to the rise of the temperature beyond 700C (general limit) in the hydration process.
Similar formation exhibit by the sulfate attack too.
Let’s key points of the formation.
- Sulfate ions entered into the concrete react with Calcium Aluminate Hydrate and form Calcium Sulfo-Aluminate Hydrate which is also called Ettringite, 3CaO.Al2O3.3CaSO4.32H2O.
- This material is distractive and expansive.
- The solid volume is greater than that of the original volume
- Grow as myriad acicular crystals – needle-shaped.
Gypsum Formation
- Sulfate ions (SO42-) may react with the Calcium Hydroxide Ca(OH)2 forms Gypsum is also known as Calcium Sulfate Dihydrate, CaSO4.2H2O.
- The formation of gypsum increases the said volume and as a result cracking and loss of elastic properties could occur.
React with Magnesium Ions
- There will be a reaction if the magnesium ions are accompanied by the sulfates.
- The magnesium ions react with the Calcium Hydroxide and produce brucite is also known as magnesium hydroxide, Mg(OH)2.
- This is low soluble and precipitates out of the solution lead to an increase in the solid volume.
- In addition, magnesium ions react with Calcium Silicate Hydrate which is the principal bonding material in the set concrete. cause loss of elastic properties of concrete.
What happens if Sulfate Attack the Concrete?
- Decalcification
Decalcification is the decomposition of hydrated calcium silicates. It is the material that maintains the principal bond.
This reaction will affect the elastic properties severely resulting in loss of strength of structural elements.
- Increase internal Volume
The internal volume of the concrete will increase when sulfate reacts to form the ettringite and gypsum forming the crystals. It generates internal expansive stresses.
- Cracking of concrete
When the internal stress exceeds the tensile strength of the concrete, concrete starts cracking. Initially, these cracks may not be visible until they developed up to the surface level.
- Corrosion of Reinforcement
Development of cracks up to the surface level could lead to expense the reinforcement to the external environment causing corrosion of reinforcements.
How to Build a Durable Structure
Design of a structure to resist the sulfate attacks can be done. We can not bring the structure somewhere else and instead, with necessary safety measures, design and construction can be done.
These steps can be identified in this process.
- Design according to the Code provisions
- Modify the Cement
- Protect the Structure Externally
Sulfate Resistance Design as per Code Provisions
Modern codes are more concerned about the durability of the structure. There are special provisions to select materials especially cement.
Let find how to achieve sulfate resistance by cement and water.
- BS EN 1008:2002 limits the sulfate content of mixing water for concrete to 2000mg/L.
This can be verified during the soil investigation. Water can be tested to know whether it is within the limit.
- Selection of Cement content, Concrete Grate, etc based on the exposure condition
A structure build in an aggressive chemical environment needs to comply with several design code requirements.
BS 8500-1:2015+A1:2016 is the latest code available to select the type of cement as per the durability requirements.
- Select the Aggressive Chemical Environment for Concrete Exposure Class ( ACEC Class) from Table A2 depending on the available sulfate content and any other.
- Nominal cover to the reinforcement could be selected from Table A10 and the same table can be used to find the DC-Class.
- Limiting values for composition and properties for concrete for each DC-Class can be obtained from Table A12. Selection of maximum water-cement ratio, minimum cement or combination content, and cement and combination type (as specified in Table A6) can be done with Table A12.
- In addition, depending on the service life and other exposure classes, Table A4 and A5 will apply.
Modify Cement
- Table A6 of BS 8500-1:2015+A1:2016 specify the cement and cement combination types. CEM I -SR 0, CEM I -SR 3, CEM IIB+SR, CEM IIIA+SR and CEM IIIB+SR are specified as sulfate resistant cement combination.
- In these cement and cement combinations, the basic components could be modified as required.
- The Tricalcium Aluminate (C3A) is kept at the minimum level for reducing the extent of reaction with sulfate ions.
Protect the Structure Externally
- As the initial step, when possible, the foundation of the structure could be placed above the groundwater table. It will avoid/minimize the groundwater touching the concrete surface.
- A waterproofing membrane could be provided to avoid the structure exposing the external environment. Generally, waterproofing is granted up to 10 years. When the structure is built for 50 years, this solution may not be the best to consider. However, there will be certain protection.
- Application of external coatings.
- Providing sacrificial layers. The thickness of the concrete can be increased allowing the chemicals to absorb to the sacrificial layer. This method may not be applicable for some construction like friction piles where the surface should be of good quality to carry the load. However, in other locations such as normal foundations, this method may be applied.