Heat of Hydration | Inevitable Cause of Concreting
Civil engineering is often a complex and multifaceted process, involving the application of mathematics, physics, and engineering principles. One variable of particular relevance for many civil engineering projects is the heat of hydration, which has both a direct and indirect effect on the overall success of a project.
This article provides an overview of the concept of the hydration, its applications, and how it can be controlled and measured to ensure a successful project.
The heat of hydration is the heat released when hydration reactions occur. This heat is created by the chemical reaction between water and cement, which produces calcium hydroxide. This can cause problems for many civil engineering projects, as it can lead to an increase in the temperature of the concrete, which can cause cracking and other structural damage.
The heat of hydration can also cause problems for the environment, as the increased temperature can lead to evaporation and condensation, which can lead to air pollution and water shortages.
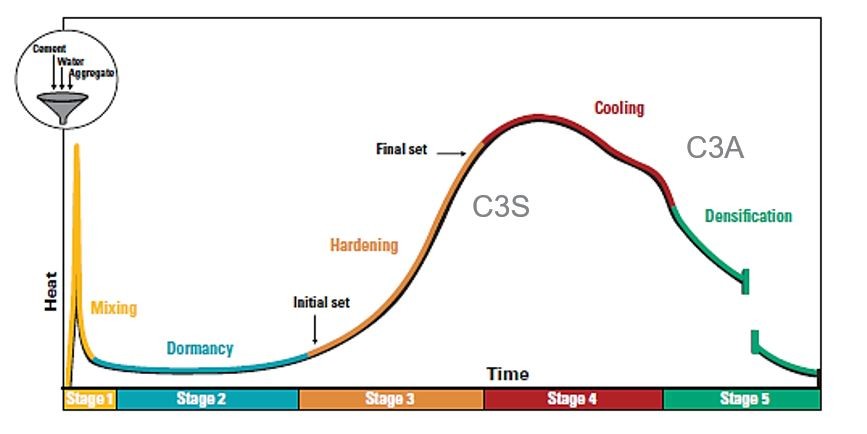
Concrete Hydration Reaction
The main component of the cement are as follows.
| Components | Percent by Weight | Chemical Formula | |
| C3S | Tricalcium silicate | 50% | 3Ca0 SiO2 |
| C2S | Dicalcium silicate | 25% | 2Ca0 SiO2 |
| C3A | Tricalcium aluminate | 10% | 3Ca0 Al2 O3 |
| C4AF | Tetracalcium aluminoferrite | 10% | 4Ca0 Al2 Fe2 O3 |
| Gypsum | 5% | CaSO4 H2O |
The strongest component of concrete is calcium silicate. The majority of the early strength is caused by tricalcium silicates (first seven days). In later stages, the slower-forming initial dicalcium silicate hydrates help to strengthen the concrete.
The heat produced by the reaction of water and Portland cement is known as the heat of hydration. The percentage of C3S and C3A in the cement has the biggest impact on heat of hydration, although it is also impacted by the water-cement ratio, fineness, and curing temperature.
Typically, the first 24 hours see the highest rate of heat liberation, and the first three days see the most heat evolution. Long-term heat generation is not a problem for the majority of concrete components, such as pavements, because this heat is dissipated into the environment.
Special Notes on Heat of Hydration
There are two main ways to control the heat of hydration: by using a lower water-to-cement ratio, or by using a plasticizer. A lower water-to-cement ratio will produce concrete that is less susceptible to cracking and damage from the hydration. A plasticizer can be used to add flexibility to the concrete, which will also help to prevent cracking.
The heat of hydration can be measured by using a calorimeter. This device measures the heat released by the hydration reaction. The calorimeter is placed in the center of the concrete mass, and the temperature is monitored over time. The result of the calorimetry is used to calculate the heat of reaction.